Abstract and Introduction
Introduction
As of March 7, 2023, a total of 30,235 confirmed and probable monkeypox (mpox) cases were reported in the United States,† predominantly among cisgender men§ who reported recent sexual contact with another man.[1] Although most mpox cases during the current outbreak have been self-limited, cases of severe illness and death have been reported.[2–4] During May 10, 2022–March 7, 2023, 38 deaths among persons with probable or confirmed mpox¶ (1.3 per 1,000 mpox cases) were reported to CDC and classified as mpox-associated (i.e., mpox was listed as a contributing or causal factor). Among the 38 mpox-associated deaths, 94.7% occurred in cisgender men (median age = 34 years); 86.8% occurred in non-Hispanic Black or African American (Black) persons. The median interval from symptom onset to death was 68 days (IQR = 50–86 days). Among 33 decedents with available information, 93.9% were immunocompromised because of HIV. Public health actions to prevent mpox deaths include integrated testing, diagnosis, and early treatment for mpox and HIV, and ensuring equitable access to both mpox and HIV prevention and treatment, such as antiretroviral therapy (ART).[5]
Data included in this report were collected during May 10, 2022–March 7, 2023. Jurisdictional health departments electronically reported confirmed and probable mpox cases and associated deaths as part of national case surveillance. Case data were shared with CDC through a standardized case report form or through the National Notifiable Diseases Surveillance System.** Additional data (e.g., clinical course, co-occurring health conditions, and treatments received) about some decedents were collected during consultations between treating clinicians and CDC clinical officers.†† Cause of death was most commonly determined by the treating health care provider and reported on the death certificate. Jurisdictional health departments shared cause-of-death data as reported on the death certificate to support classification of deaths. Deaths were classified as mpox-associated if mpox was listed on Part I or Part II of the death certificate (chain of events that directly caused the death or significant conditions contributing to death, respectively). Deaths were classified as non–mpox-associated if mpox was not listed on the death certificate, or if mpox appeared to be incidental to death. Deaths were classified as being under investigation§§ if jurisdictions were reviewing the cause-of-death with health care providers at the time of this analysis.
Descriptive statistics on demographics (e.g., age, gender identity, race and ethnicity, and state of residence) and clinical characteristics (i.e., treatments received and timing of treatments) were calculated for all patients with an mpox-associated death and were compared between decedents and patients who are presumed to have survived after confirmed or probable mpox (survivors). SAS statistical software (version 9.4; SAS Institute) was used for all analyses. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.¶¶
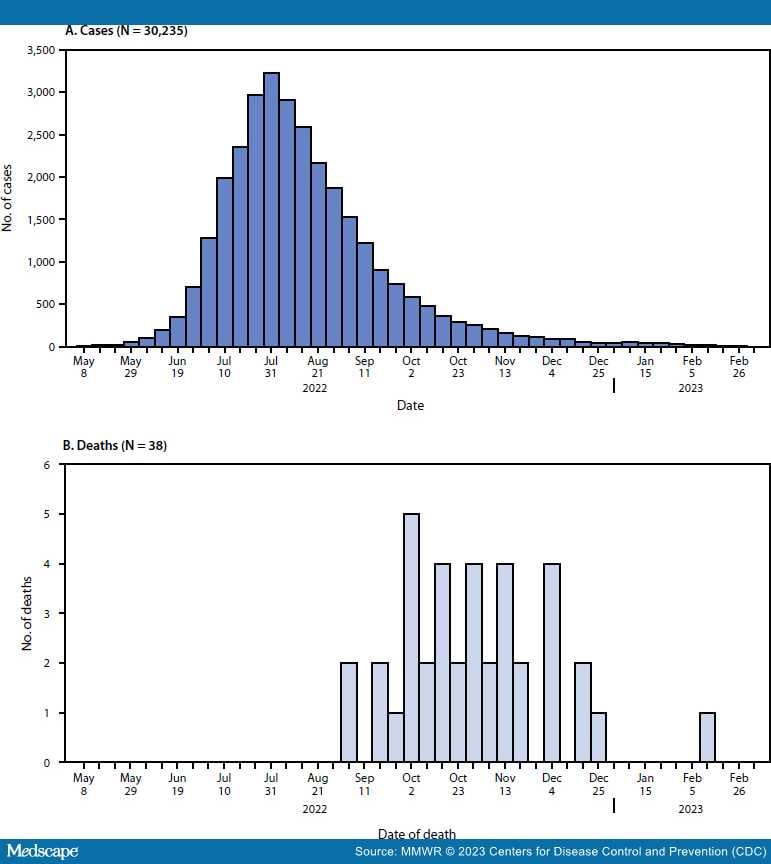
CDC received reports of 52 deaths among persons with confirmed or probable mpox. Thirty-eight deaths (73.1%) were classified as mpox-associated, three (5.8%) were non–mpox-associated,*** and 11 (21.2%) deaths remain under investigation. Among the 38 mpox-associated deaths, 25 (65.8%) occurred during October–November 2022 (Figure).
Figure.
Weekly confirmed and probable* mpox cases (A)† and mpox-associated deaths (B),§ by week — United States, May 10, 2022—March 7, 2023
*https://www.cdc.gov/poxvirus/mpox/clinicians/case-definition.html
†The earliest date available regarding the case, beginning with date of illness or rash onset, diagnosis date, positive laboratory test report date, CDC call center reporting date, or case data entry date into the National Notifiable Diseases Surveillance System.
§Date of death as reported on the death certificate.
The median age of decedents was 34 years (range = 22–58 years), and 36 (94.7%) were cisgender men (Table 1). A higher proportion of decedents than survivors were Black (86.8% versus 32.9%). Nearly one half of decedents (47.4%) resided in the U.S. Census Bureau South Region††† compared with 39.4% of survivors who lived in this region. Nine of the 10 decedents with recent sexual or intimate contact information had sexual or intimate contact with cisgender men in the 3 weeks preceding symptom onset. Two decedents reported nonsexual close contact exposures (e.g., co-sleeping and caring for a household member) to persons with mpox. Five of 11 decedents with available data were experiencing homelessness. Among 87% of decedents and 45% of survivors with available information, HIV infection was more prevalent among decedents than survivors (93.9% versus 38.3%).
Among 27 (71.0%) decedents with available clinical data, the median interval from symptom onset to death was 68 days (range = 1–146 days; IQR = 50–86 days), and 87.0% (20 of 23) were admitted to an intensive care unit during hospitalization (Table 2). Overall, 25 (92.6%) of 27 decedents with available treatment information received mpox-directed medical therapeutics§§§ at some point during their clinical care, including 25 who received tecovirimat, 18 of 24 who received vaccinia immunoglobulin intravenous (VIGIV), nine of 22 who received cidofovir, and six of 15 who received brincidofovir. Among the 25 decedents who received tecovirimat, 15 (60.0%) received it within 3 days of mpox diagnosis; however, for six (24.0%) decedents, tecovirimat was not administered until ≥3 weeks after diagnosis (median overall interval = 2 days; IQR = 0–20 days). Among 24 decedents with available data, all had lesions described as necrotic, diffuse, or worsened after a 14-day course of tecovirimat.
Two decedents did not receive any mpox therapeutics based on clinician discretion; in one case because of concerns regarding contraindication associated with other comorbidities. The other decedent was on ART for HIV with an undetectable viral load at the time of initial assessment for mpox care. Approximately 1 month later, at a wellness check, the patient was found deceased with diffuse lesions characteristic of mpox. Seven of 27 decedents for whom information was available refused therapeutic or intravenous medications or left the hospital against medical advice at some point during their clinical course of treatment. Among 24 decedents with information on receipt of steroids, more than one half (54.2%) received steroids for mpox complications or concerns about immune reconstitution inflammatory syndrome (IRIS), a hyperinflammatory response that can occur in patients with HIV during the first 6 months of ART.[6]
Nearly all decedents with complete data on HIV infection were HIV-positive (93.9%; 31 of 33). Among 24 decedents with HIV and available data, all (100%) had CD4 counts <200 cells/mm3; 23 (95.8%) had CD4 counts <50 cells/mm3. Among the two immunocompromised decedents who did not have HIV, one was presumed to have been immunocompromised as a consequence of undiagnosed diabetes; the patient experienced diabetic ketoacidosis, a severe life-threatening complication of diabetes, at the time of mpox diagnosis. The second decedent was severely immunocompromised because of a recent renal transplant complicated by acute rejection.
Only two of the 25 persons with HIV (8.0%) reported taking ART before mpox diagnosis; in one of these decedents, HIV was poorly controlled. ART was initiated for 19 of the 20 decedents who were not receiving ART, including one decedent who received a diagnosis of HIV infection 5 days after the mpox diagnosis. One decedent refused treatment for advanced HIV. ART treatment status was not known for three of the 25 decedents with HIV. ART was either delayed or interrupted for seven decedents because of clinician concerns about IRIS.
Morbidity and Mortality Weekly Report. 2023;72(15):404-410. © 2023 Centers for Disease Control and Prevention (CDC)